Can Myopia Be Reversed? - A Study on the Role of Atropine Eye drops in Arresting Myopia Progression
Abstract
Purpose:
- To investigate the effect of atropine in retarding progression of myopia.
- To compare rates of retardation of myopia progression using 0.05% atropine and 0.1% atropine.
Methods:
This is a hospital based prospective study. Patients were selected from children, between 6-18 yrs of age, visiting the outpatient department of our hospital, with spherical equivalent (SE) =>-1D in both the eyes (with spherical equivalent (SE) progression rate >= 0.5D/year). Groups with 0.05% atropine eyedrops, 0.1% atropine eyedrops and control were allocated randomly. Myopia progression was measured by change in spherical equivalent and axial length at baseline and every 3 months till 1 year.
Results:
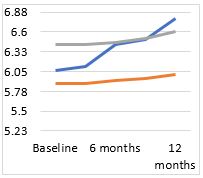
In our study, a total of 48 eyes of 24 children were included, out of which 83.33% were in age group 6-12years; rest in 13-18years age group. The mean change in spherical equivalent in our study after one year was -0.72±0.21D, -0.11±0.096D and -0.19±0.18D in placebo, 0.1% and 0.05% atropine groups respectively. Also, the mean change in axial length our study after one year was 0.45±0.15mm, 0.13±0.20mm and 0.11±0.02mm in placebo, 0.1% and 0.05% atropine groups respectively. Change in both parameters were found to be statistically significant.
Conclusion:
We conclude that night time application of 0.05% and 0.1% atropine eyedrops is efficacious in retarding progressive myopia in Indian eyes. Atropine eyedrops were better tolerated in pre-adolescent children. Drop out rate was more in the adolescent population, which may be due to more amount of near work.
KEYWORDS: atropine, axial length, myopia, spherical equivalent
Downloads
References
Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012 May 5;379(9827):1739-48. doi: 10.1016/S0140-6736(12)60272-4.
Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012 Jan;32(1):3-16. doi: 10.1111/j.1475-1313.2011.00884.x.
Sun J, Zhou J, Zhao P, Lian J, Zhu H, Zhou Y, et al. High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai. Invest Ophthalmol Vis Sci. 2012 Nov 1;53(12):7504-9. doi: 10.1167/iovs.11-8343.
Williams KM, Bertelsen G, Cumberland P, Wolfram C, Verhoeven VJ, Anastasopoulos E, et al. Increasing Prevalence of Myopia in Europe and the Impact of Education. Ophthalmology. 2015 Jul;122(7):1489-97. doi: 10.1016/j.ophtha.2015.03.018.
Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. 2002 Apr;109(4):704-11. doi: 10.1016/s0161-6420(01)01024-7.
Walline JJ, Lindsley K, Vedula SS, Cotter SA, Mutti DO, Twelker JD. Interventions to slow progression of my-opia in children. Cochrane Database Syst Rev. 2011 Dec 7;(12):CD004916. doi: 10.1002/14651858.CD004916.
Sun Y, Xu F, Zhang T, Liu M, Wang D, Chen Y, et al. Orthokeratology to control myopia progression: a me-ta-analysis. PLoS One. 2015 Apr 9;10(4):e0124535. doi: 10.1371/journal.pone.0124535.
Gwiazda J. Treatment options for myopia. Optom Vis Sci. 2009 Jun;86(6):624-8. doi: 10.1097/OPX.0b013e3181a6a225.
Donders, Franciscus Cornelis, and William Daniel Moore. On the anomalies of accommodation and refrac-tion of the eye: With a preliminary essay on physiological dioptrics. Vol. 22. New Sydenham Society, 1864.
Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, et al. Atropine for the treatment of child-hood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012 Feb;119(2):347-54. doi: 10.1016/j.ophtha.2011.07.031.
Saw SM, Shih-Yen EC, Koh A, Tan D. Interventions to retard myopia progression in children: an evidence-based update. Ophthalmology. 2002 Mar;109(3):415-21; discussion 422-4; quiz 425-6, 443. doi: 10.1016/s0161-6420(01)00972-1.
Prepas SB. Light, literacy and the absence of ultraviolet radiation in the development of myopia. Med Hy-potheses. 2008;70(3):635-7. doi: 10.1016/j.mehy.2007.07.023.
Kothari M, Rathod V. Efficacy of 1% atropine eye drops in retarding progressive axial myopia in Indian eyes. Indian J Ophthalmol. 2017 Nov;65(11):1178-1181. doi: 10.4103/ijo.IJO_418_17.
Salazar M, Shimada K, Patil PN. Iris pigmentation and atropine mydriasis. J Pharmacol Exp Ther. 1976 Apr;197(1):79-88.
Anderson HA, Bertrand KC, Manny RE, Hu YS, Fern KD. Comparison of two drug combinations for dilat-ing dark irides. Optom Vis Sci. 2010 Feb;87(2):120-4. doi: 10.1097/OPX.0b013e3181cc8da3.
Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myo-pia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009 Mar;116(3):572-9. doi: 10.1016/j.ophtha.2008.10.020.
Huang J, Wen D, Wang Q, McAlinden C, Flitcroft I, Chen H, et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-analysis. Ophthalmology. 2016 Apr;123(4):697-708. doi: 10.1016/j.ophtha.2015.11.010.
Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014 Feb;157(2):451-457.e1. doi: 10.1016/j.ajo.2013.09.020.
Siatkowski RM, Cotter S, Miller JM, Scher CA, Crockett RS, Novack GD; US Pirenzepine Study Group. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: a 1-year, multicenter, double-masked, placebo-controlled parallel study. Arch Ophthalmol. 2004 Nov;122(11):1667-74. doi: 10.1001/archopht.122.11.1667.
Tigges M, Iuvone PM, Fernandes A, Sugrue MF, Mallorga PJ, Laties AM, Stone RA. Effects of muscarinic cholinergic receptor antagonists on postnatal eye growth of rhesus monkeys. Optom Vis Sci. 1999 Jun;76(6):397-407. doi: 10.1097/00006324-199906000-00020.
Lind GJ, Chew SJ, Marzani D, Wallman J. Muscarinic acetylcholine receptor antagonists inhibit chick scleral chondrocytes. Invest Ophthalmol Vis Sci. 1998 Nov;39(12):2217-31.
Copyright (c) 2022 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative



















 Therapoid
Therapoid

