Outcome following single intravitreal injection of ranibizumab in branch retinal vein occlusion patients: a single centre experience
Abstract
Objective:To find out outcome following single intravitreal injection of ranibizumab in branch retinal vein occlusion (BRVO) patients in a government hospital in the capital of Odisha.
Methods: This study was a prospective interventional study of 18 months duration, done from August 2017 to February 2019 which included 21 cases of BRVO. Thorough history was taken and detail ophthalmological evaluation was done. Best corrected visual acuity (BCVA) was examined with Snellen’s chart and central macular thickness (CMT) was measured using optical coherence tomography (OCT). Routine blood tests were done. Intravitreal injection of ranibizumab 0.5 mg in 0.05 ml was given. BCVA and CMT was measured at 1 day, 1 week, 1 month and 3 months follow up visit.
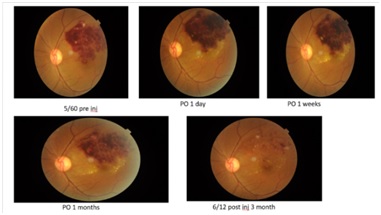
Results: Mean age was 55.29 ± 16.86 yrs. Male: female ratio was 3.2:1. 33. About 3% patients presented within 1 month of onset of symptoms. Diastolic blood pressure of ≥ 90 was found in 81% patients. 90.5% showed improvement at 1 week post-operatively.At post-operative day 1 CMT was significantly decreased (p= 0.0011). Reduction in mean CMT at post-operative day 7 was >270 µ.
Conclusion: Single intravitreal injection of ranibizumab 0.5 mg in 0.05 mlcauses significant increase in vision and significant reduction of central macular thickness in patients with branch retinal vein occlusion.
Downloads
References
2. Hayreh SS. Classification of central retinal vein occlusion. Ophthalmology 1983; 90:458–474The eye disease case-control study group. Risk factors for branch retinal vein occlusion. American journal of ophthalmology. September 1993; 116(3): 286-96.
3. Brown BA, Marx JL, Ward TP, et al. Homocysteine: a risk factor for retinal venous occlusive disease. Ophthalmology. 2002 Feb;109(2):287-90.[pubmed]
4. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994 Dec 1;331(22):1480-7. DOI:10.1056/NEJM199412013312203.[pubmed]
5. Ozaki H, Hayashi H, Vinores SA, et al. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp Eye Res. 1997 Apr;64(4):505-17. DOI:10.1006/exer.1996.0239.[pubmed]
6. Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006 Oct;26(8):859-70. DOI:10.1097/01.iae.0000242842.14624.e7.[pubmed]
7. Rayess N, Rahimy E, Shah CP, et al. Incidence and clinical features of post-injection endophthalmitis according to diagnosis. Br J Ophthalmol. 2016 Aug;100(8):1058-61. doi: 10.1136/bjophthalmol-2015-307707. Epub 2015 Nov 19.
8. Kida T, Tsujikawa A, Muraoka Y, Harion S, Osaka R, Murakami T, Ooto S, Suzuma K, Maroishita S, Fukumoto M, Suzuki H, Ikeda T. Cotton wool spots after anti-vascular endothelial growth factortherapy for macular edema associated with central retinal vein occlusion. Ophthalmologica. 2016;235(2):106–113.
9. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010 Jun;117(6):1102-1112.e1. doi: 10.1016/j.ophtha.2010.02.021. Epub 2010 Apr 15.[pubmed]
10. Klein R, Klein BE, Moss SE, et al. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133-41; discussion 141-3.[pubmed]
11. Osaka R, Muraoka Y, Miwa Y, et al. Anti-Vascular Endothelial Growth Factor Therapy for Macular Edema following Central Retinal Vein Occlusion: 1 Initial Injection versus 3 Monthly Injections. Ophthalmologica. 2018;239(1):27-35. doi: 10.1159/000479049. Epub 2017 Sep 26.[pubmed]
12. Ach T1, Hoeh AE, Schaal KB, et al. Predictive factors for changes in macular edema in intravitreal bevacizumab therapy of retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2010 Feb;248(2):155-9. doi: 10.1007/s00417-009-1167-6. Epub 2009 Sep 9.[pubmed]
13. Shin HJ, Chung H, Kim HC. Association between integrity of foveal photoreceptor layer and visual outcome in retinal vein occlusion. Acta Ophthalmol. 2011 Feb;89(1):e35-40. doi: 10.1111/j.1755-3768.2010.02063.x.Epub 2010 Dec 14.[pubmed]
14. Miwa Y, Muraoka Y, Osaka R, Ooto S, Murakami T, Suzuma K, Takahashi A, Iida Y, Yoshimura N, Tsujikawa A. Ranıbızumab for macular edema after branch retinal vein occlusıon one initial ınjection versus three monthly ınjections. Retina. 2017;37(4):702–709. [pubmed]
15. Prager F, Michels S, Kriechbaum K, et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol. 2009 Apr;93(4):452-6. doi: 10.1136/bjo.2008.141085. Epub 2008 Dec 15.
Copyright (c) 2019 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative



















 Therapoid
Therapoid

