To study the effect of Rebamipide 2% ophthalmic suspension in Dry eye
Abstract
Purpose: Current available therapies such as lubricants and anti-inflammatory drugs alleviate symptoms and reduce signs of dry eye. Various drugs have been developed to treat the underlying cause of disease .One such drug is Rebamipide 2% ophthalmic suspension. Our study aims to study the efficacy of Rebamipide 2% ophthalmic suspension in treating dry eye.
Material and methods: It was a Prospective interventional study in which 60 patients were divided into two groups. Group A included those 30 cases which were subjected to rebamipide 2% (q.i.d) and 0.3% Hydroxypropylmethyl cellulose (q.i.d). Group B included those 30 cases, which were subjected to 0.3% Hydroxypropylmethyl cellulose (q.i.d) alone. Follow up was done at an interval of two weeks till twelve weeks. Beside recording improvement in symptoms following tests were performed at each visit - Schirmer’s test, Fluoresce in staining test, Fluoresec in tear break up time (TBUT).
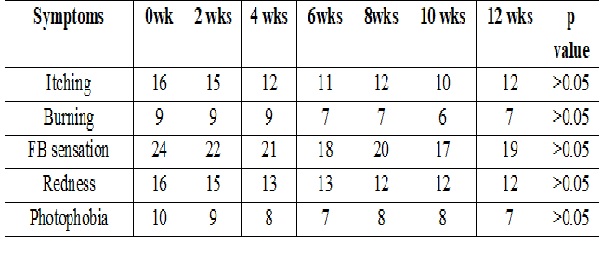
Results: Cases treated with Rebamipide 2% eye drops showed a statistically significant improvement in both subjective and objective measures.There was a significant improvement in grittiness besides significant improvement in schirmers (p<0.001)), TBUT (p<0.01) and Fluorescien staining of cornea (p<0.001). The control group showed no significant difference compared to baseline.
Conclusion: Our data suggests that rebamipide 2% ophthalmic suspension is effective in treating dry eye.
Downloads
References
2. Ueta M, Sotozono C, Yokoi N, Kinoshita S. Rebamipide suppresses PolyI:C-stimulated cytokine production in human conjunctival epithelial cells. J OculPharmacolTher 2013 Sep; 29(7): 688–93.[pubmed]
3. Kimura K, Morita Y, Orita T, Haruta J, Takeji Y, Sonoda KH. Protection of human corneal epithelial cells from TNF-alpha-induced disruption of barrier function by rebamipide. Invest Ophthalmol Vis Sci 2013 Apr 17; 54(4): 2572–760.[pubmed]
4. Kashima T, Akiyama H, Miura F, Kishi S. Resolution of persistent corneal erosion after administration of topical rebamipide. Clin Ophthalmol 2012; 6: 1403–6.[pubmed]
5. “Methodologies to diagnose and monitor dry eye disease: report of the diagnostic methodology subcommitteeof the internationaldryeyeworkshop. TheOcularSurface 2007 Apr;5(2):108-52. [pubmed]
6. Rios JD, Shatos M, Urashima H, Tran H, Dartt DA. OPC-12759 increases proliferation of cultured rat conjunctival goblet cells. Cornea 2006jun; 25(5): 573–81.[pubmed]
7.Sugai S, Takahashi H, Ohta S, Nishinarita M, Takei M, Sawada S, Yamaji K, Oka H, Umehara H, Koni I, Sugiyama E, Nishiyama S, Kawakami A. Efficacy and safety of rebamipide for the treatment of dry mouth symptoms in patients with Sjogren’s syndrome: a double-blind placebo-controlled multicenter trial. Mod Rheumatol 2009; 19(2): 114–24.[pubmed]
8. H. Tanaka, K. Fukuda, W. Ishida, Y. Harada, T. Sumi, and A. Fukushima. Rebamipide increases barrier function and attenuates TNFα-induced barrier disruption and cytokine expression in human corneal epithelial cells..Br J Ophthalmol.2013;97(7) : 912–916.[pubmed]
9. S. Kinoshita, K. Oshiden, S. Awamura, H. Suzuki, N. Naka- michi, and N. Yokoi .A randomized, multicenter phase 3 study comparing 2% rebamipide (OPC-12759) with 0.1% sodium hyaluronate in the treatment of dry eye. Ophthalmology. 2013 Jun; 120(6): 1158-65.[pubmed]
10. “Acucela and Otsuka Pharmaceutical Announce the Initiation of a Phase 3 Clinical Trial to Evaluate Rebamipide Ophthalmic Suspension in Patients with Dry Eye Syndrome,” http://www .acucela.com/Read-About-Us/Press-Releases.
11. M. S. Norn.Desiccation of the precorneal tear film, corneal wetting time. Acta Ophthalmologica.1969 ;47(4) : 865–880.[pubmed]
12. S. Kinoshita, S. Awamura, K. Oshidenetal..Rebamipide (OPC-12759) in the treatment of dry eye: a randomized, double-masked, multicenter, placebo-controlled phase II study. Ophthalmology. 2012 Dec;119(12): 2471–2478.[pubmed]
13. Koh S, Inoue Y, Sugmimoto T, Maeda N, Nishida K. Effect of rebamipide ophthalmic suspension on optical quality in the short break-up time type of dry eye. Cornea. 2013 Sep;32(9):1219–1223.
Copyright (c) 2018 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative



















 Therapoid
Therapoid

