Comparative analysis of efficacy and safety of Bilastine 20 mg and Levocetirizine 5 mg in the treatment of Allergic Rhinoconjunctivitis
Abstract
Objective: The present study was conducted to assess the efficacy and safety of bilastine 20 mg and compare the results with that of levocetirizine 5 mg in the treatment of allergic rhinoconjunctivitis.
Material and Methods: It was a prospective study conducted in the Department of Ophthalmology and Department of Otorhinolaryngology at a tertiary institute of southern Rajasthan, India during the period of 6 months from September 2019 to February 2020. 100 patients of chronic allergic rhinoconjunctivitis were included in the study, of which 50 were treated with Bilastine 20 mg, and the rest 50 patients were treated with levocetirizine 5 mg. The primary assessment was done by calculating the total symptom score (TSS) before and after the 7th and 14th post-treatment day.
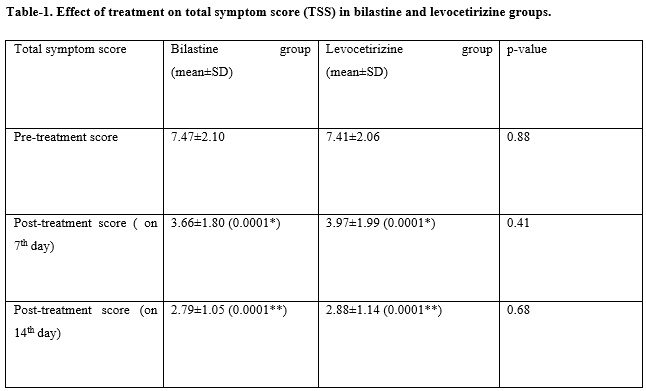
Results: The age of the patients ranges from 10 years to 65 years with a mean age of 32±5.2 years. The primary efficacy parameter for assessment was a reduction in total symptom score (TSS). Both bilastine 20 mg and levocetirizine 5 mg significantly reduced the TSS on the 7th and 14th post-treatment days (p-value< 0.001). There was no significant difference between TSS of bilastine and levocetirizine after 7 days (p-value= 0.41) and after 14 days treatment (p-value= 0.68). Adverse events were reported by 10% of patients in the bilastine group and by 38% of patients in the levocetirizine group.
Conclusion: Bilastine is a selective H1 antihistamine with good efficacy and excellent safety profile and it is highly recommended to use it as a first-line treatment for allergic rhinoconjunctivitis.
Downloads
References
Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss MS. World Allergy Organization (WAO) white book on allergy: update 2013. Milwaukee, USA: World Allergy Organization; 2013. p. 11–13; 27–31, 60–63. Available at https://www.worldallergy.org/UserFiles/file/WhiteBook2-2013-v8.pdf.
Bousquet J, van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5):S147–S334. doi: 10.1067/mai.2001.118891.
Rutkowski R, Kosztyla-Hojna B, Rutkowska J. Allergic rhinitis – an epidemiological, economical and social problem of the XXI century. Pneumenol Alergol Pol 2008;76(5):348–352.
Van Cauwenberge P, Bachert C, Passalacqua G, Bousquet J, Canonica GW, Durham SR et al. Consensus statement on the treatment of allergic rhinitis. Allergy 2000;55(2):116–134. doi: 10.1034/j.1398-9995.2000.00526.x.
Weir E. The burden of rhinitis: nothing to sniff at. CMAJ September 2003;169(7):694.
Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128(6):1139-1150.e4. doi: 10.1016/j.jaci.2011.09.005.
Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466–476. doi: 10.1016/j.jaci.2010.06.047.
Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA2LEN/EDF/ WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–1414. doi: 10.1111/all.13397.
Bosma R, van den Bor J, Vischer HF, Labeaga L, Leurs R. The long duration of action of the second generation antihistamine bilastine coincides with its long residence time at the histamine H(1) receptor. Eur J Pharmacol. 2018;5(838):107–111. doi: 10.1016/j.ejphar.2018.09.011.
Camelo-Nunes IC, Sol_e D. Allergic rhinitis: indicators of quality of life. J Bras Pneumol. 2010;36(1):124-133. doi: 10.1590/s1806-37132010000100017.
Maurer M, Abuzakouk M, Berard F, Canonica W, Oude Elberink H, Amau AG, et al. The burden of chronic spontaneous urticaria is substantial: real-world evidence from ASSURE-CSU. Allergy. 2017;72(12):2005–2016. doi: 10.1111/all.13209.
Kirmaz C, Aydemir O, Bayrak P, Yuksel H, Ozenturk O, Degirmenci S. Sexual dysfunction in patients with allergic rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2005;95(6):525–529. doi: 10.1016/S1081-1206(10)61013-7.
Maurer M, Weller K, Bindslev‐Jensen C, Giménez‐Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011;66(3):317–330. doi: 10.1111/j.1398-9995.2010.02496.x.
Juniper EF, Guyatt GH, Dolovich J. Assessment of quality of life in adolescents with allergic rhinoconjunctivitis: development and testing of a questionnaire for clinical trials. J Allergy Clin Immunol. 1994;93(2):413–423. doi: 10.1016/0091-6749(94)90349-2.
Keil T, Bockelbrink A, Reich A, Hoffmann U, Kamin W, Forster J, et al. The natural history of allergic rhinitis in childhood. Pediatr Allergy Immunol. 2010;21(6):962–969. doi: 10.1111/j.1399-3038.2010.01046.x.
Blaiss MS, Hammerby E, Robinson S, Kennedy-Martin T, Buchs S. The burden of allergic rhinitis and allergic rhinoconjunctivitis on adolescents: a literature review. Ann Allergy Asthma Immunol. 2018;121(1):43–52.e3. doi: 10.1016/j.anai.2018.03.028.
Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155(1):145-151. doi: 10.1111/j.1365-2133.2006.07185.x.
Fitzsimons R, van der Poel LA, Thornhill W, et al. Antihistamine use in children. Arch Dis Child Educ Pract Ed. 2015;100(3):122-131. doi: 10.1136/archdischild-2013-304446.
Bousquet J, Van Cauwenberge P, Bachert C, Canonica GW, Demoly P, Durham SR, et al. Requirements for medications commonly used in the treatment of allergic rhinitis. European Academy of Allergy and Clinical Immunology (EAACI), Allergic Rhinitis and its Impact on Asthma (ARIA). Allergy. 2003;58(3):192–197. doi: 10.1034/j.1398-9995.2003.00054.x.
Kuna P, Bachert C, Nowacki Z, van Cauwenberge P, Agache I, Fouquert L, et al. Efficacy and safety of bilastine 20 mg compared with cetirizine 10 mg and placebo for the symptomatic treatment of seasonal allergic rhinitis: A randomized, double-blind, parallelgroup study. Clin Exp Allergy. 2009;39(9):1338–1347. doi: 10.1111/j.1365-2222.2009.03257.x.
Bachert C, Kuna P, Sanquer F, Ivan P, Dimitrov V, Gorina MM, et al. Comparison of the efficacy and safety of bilastine 20 mg vs desloratadine 5 mg in seasonal allergic rhinitis patients. Allergy. 2009;64(1):158-165. doi: 10.1111/j.1398-9995.2008.01813.x.
Dávila I, Sastre J, Mullol J, Montoro J, Jáuregui I, Ferrer M, et al. Effect of bilastine upon nasal obstruction. J Investig Allergol Clin Immunol. 2011;21(3):2-8.
Bartra J, Mullol J, Montoro J, Jáuregui I, del Cuvillos A, Dávila I, et al. Effect of bilastine upon the ocular symptoms of allergic rhinoconjunctivitis. J Investig Allergol Clin Immunol. 2011;21(3):24-33.
Zuberbier T, Oanta A, Bogacka E, Medina I, Wesel F, Uhl P, et al, Bilastine International Working Group. Bilastine International Work-ing Group. Comparison of the efficacy and safety of bilastine 20 mg vs levocetirizine 5 mg for the treatment of chronic idiopathic urticaria: a multi-centre, double-blind, randomized, placebo controlled study. Allergy. 2010;65(4):516-528. doi: 10.1111/j.1398-9995.2009.02217.x.
Copyright (c) 2021 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative



















 Therapoid
Therapoid

